Please take some time to thoughtfully reply to the end of the year survey. This will provide feedback that should help me improve the course next year. Thank you.
End of year survey
End of year survey
Unit Video Playlists
|
Recommended Review Videos
|
 |
| pH scale |
 |
| Universal |
 |
 |
| Waco Texas Ammonium Nitrate Explosion |
 |
| 3-D models of alka-seltzer CO2 reaction |
 |
| explosion = rapid expansion of gases |
 |
| limewater reaction atomic movement |
 |
| molecules. |
 Period 1 Lab 8.2 spreadsheet
Period 1 Lab 8.2 spreadsheet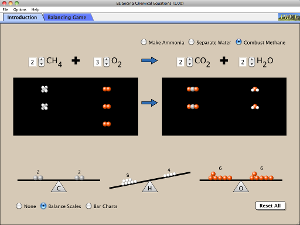
 |
| periodic table of ions |