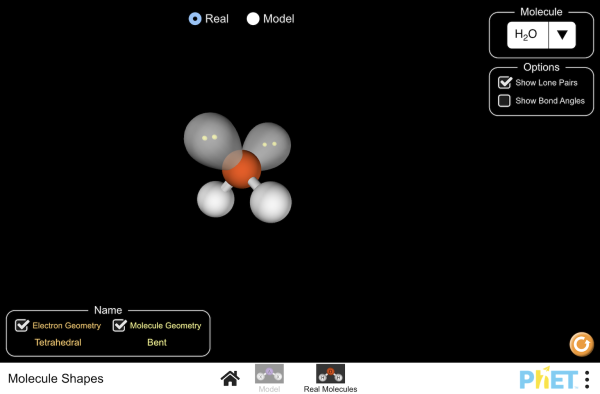
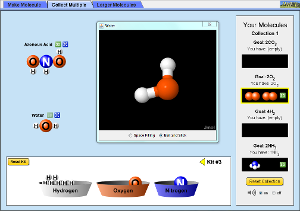
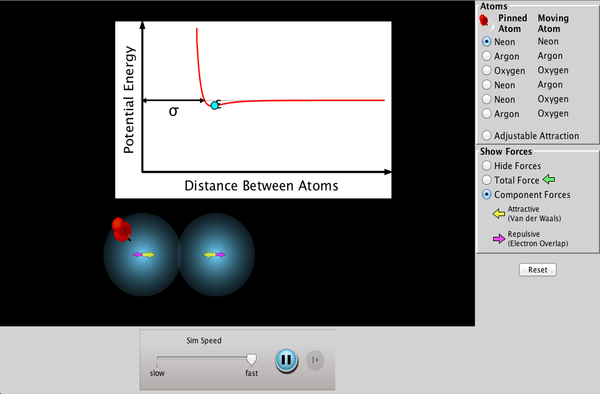
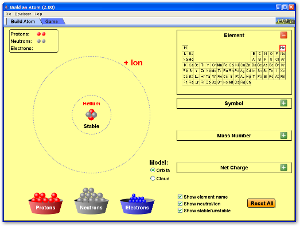
Chemical bonding content is in chapter 6 (complete review 15 to 49 odd) and chapter 7 (complete review 1 to 13 and 23 to 37 odd). This unit will last until just after winter break. Study a bit at a time. Work on memorizing ions (p 205 and p 210) for nomenclature.
FINAL EXAM REVIEW... In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts... A good review channel for the final (watch atomic structure, periodicity and bonding SL videos)
DUE DATES
Chapter 6 review 15 to 49 odd and chapter 7 1 to 13 and 23 to 37 odd
Worksheet 6.1 Dot Diagrams due 12/10
Lab 6.1 Chemical Bonding due 12/8
Candy Lab Chemistry Post lab due 12/18
Lab 7.1 Ionic Formulas due 1/11
Worksheet 7.1 Nomenclature
Worksheet 7.5
Lab 7.2: Percent Composition of Potassium Chlorate
ONLINE
Chemspider database and 3D structure finder
Bonding Crash Course
Lewis electron dot diagrams Brightstorm
Polar vs NonPolar molecules Crash Course
Bonding models and lewis structures Crash Course
Solids Crash Course
Network Solids Crash Course
Intro to writing ionic formulas
Ionic nomenclature Brightstorm
Naming acids Brightstorm
Nomenclature1 Crash Course
VSEPR theory Brightstorm
VSEPR theory Oregon State University
VSEPR theory (more advanced) Bozeman Science
lewis/valence dot periodic table image
Sugar manufacturing video
Chemist tree...whee...
Chemical Bonding SLIDES Presentation
Chemical Bonding and VSEPR SLIDES Presentation
Bonding playlist Thornley
FINAL EXAM REVIEW... In addition to study of the text and your class notes you should consider the internet, especially YouTube chemistry channels, an essential part of learning chemistry well. WATCH THE VIDEOS... you will slowly build important concepts... A good review channel for the final (watch atomic structure, periodicity and bonding SL videos)
DUE DATES
 |
| periodic table of ions |
Chapter 6 review 15 to 49 odd and chapter 7 1 to 13 and 23 to 37 odd
Worksheet 6.1 Dot Diagrams due 12/10
Lab 6.1 Chemical Bonding due 12/8
Candy Lab Chemistry Post lab due 12/18
Lab 7.1 Ionic Formulas due 1/11
Worksheet 7.1 Nomenclature
Worksheet 7.5
Lab 7.2: Percent Composition of Potassium Chlorate
ONLINE
Chemspider database and 3D structure finder
Lewis electron dot diagrams Brightstorm
Polar vs NonPolar molecules Crash Course
Bonding models and lewis structures Crash Course
Solids Crash Course
Network Solids Crash Course
Intro to writing ionic formulas
Ionic nomenclature Brightstorm
Naming acids Brightstorm
Nomenclature1 Crash Course
VSEPR theory Brightstorm
VSEPR theory Oregon State University
VSEPR theory (more advanced) Bozeman Science
lewis/valence dot periodic table image
Sugar manufacturing video
Chemist tree...whee...
Chemical Bonding SLIDES Presentation
Chemical Bonding and VSEPR SLIDES Presentation
Bonding playlist Thornley